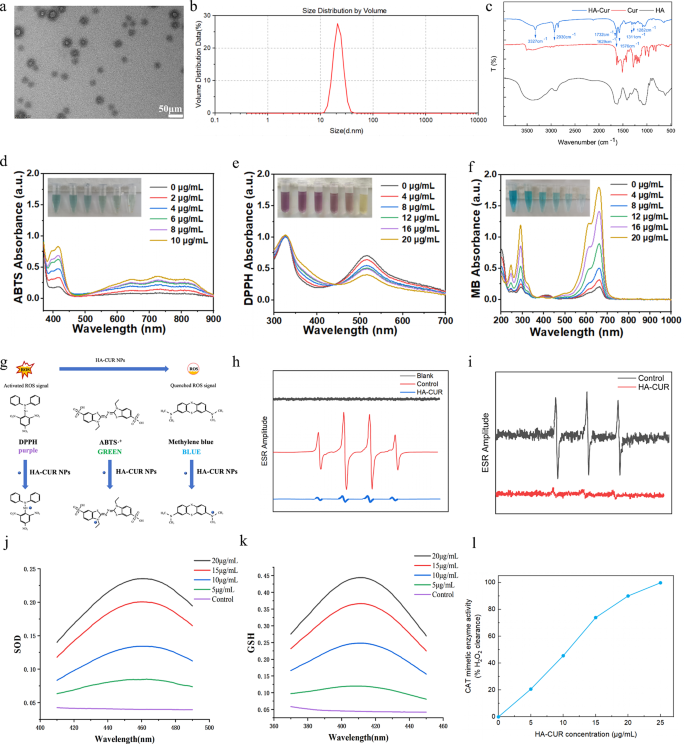
Synthesis and characterization of HA-CUR NPs
HA-CUR NPs were synthesized via esterification between the carboxyl group of HA and the hydroxy group of CUR through a straightforward and dependable method. In particular, CUR was dissolved in a reaction system containing HA, DCC, and DMAP to form HA-CUR NPs. After 24 h of stirring, the resulting mixture turned dark, suggesting the effective combination of HA and CUR. The mixture was subsequently dialyzed and centrifuged to obtain HA-CUR NPs with superior aqueous dispersion. The micromorphology of the HA-CUR NPs was observed via TEM. As shown in Fig. 1a, HA-CUR NPs were generally in the form of uniformly distributed ultrafine particles with a size of 22.4 ± 8.7 nm. The average hydrodynamic dimension of the HA-CUR NPs was subsequently measured via DLS and was comparable to the result determined via TEM (Fig. 1b). We subsequently determined the structure of the HA-CUR NPs by measuring their 1H NMR spectra. As shown in Figure S1, the peaks of HA-CUR at 1.5–2.0 ppm and 6.5–7.0 ppm were attributed to the acetyl peak of HA (- NHCOCH3) and the aromatic proton of CUR, respectively. Furthermore, the infrared intensities of the HA, CUR, and HA-CUR NPs were analyzed via FTIR to verify that the HA-CUR NPs were successfully synthesized. As shown in Fig. 1c, compared with the infrared spectra of HA and CUR, the apparent absorption peak at 3327 cm-1 was the absorption band of the -OH group in the curcumin structure; the obvious absorption peak at 2930 cm-1 was the absorption band of the double C = O group in curcumin; the new absorption peak at 1576 cm-1 was attributed to the vibration of the C = C within the benzene ring of curcumin; and the new absorption peak at 1732 cm-1 was the absorption band of the newly formed C = O double bond in the ester. Moreover, the HA-CUR NPs were dissolved in three solvents: water, ethanol and 0.9% NaCl. The UV‒vis absorbance spectra indicated that there was no marked change at 0 and 7 days (Figure S2). These results were consistent with the findings of Manju S, indicating the successful synthesis of HA-CUR NPs.
Characterization, ROS scavenging ability, and enzyme-mimicking activities of HA-CUR NPs. (a) Transmission electron microscopy images of HA-CUR NPs. (b) Dynamic light scattering data of HA-CUR NPs. (c) FTIR spectra of the HA, CUR, and HA-CUR NP samples. (d) UV‒Vis absorbance showing the ABTS radical scavenging ability of HA-CUR NPs. (e) UV‒Vis absorbance showing the DPPH radical scavenging ability of HA-CUR NPs. (f) UV‒Vis absorbance image illustrating the ability of HA-CUR NPs to scavenge ·OH to protect MB. (g) Illustration of the ROS scavenging process. (h) ESR spectra of DMPO indicating ·OH capture with or without HA-CUR NPs. (i) ESR spectra of TEMPO indicating single oxygen capture with or without HA-CUR NPs. (j) SOD-like activity of HA-CUR NPs. (WST-8 kit assay) (n = 3 for each group). (k) GSH-like activity of HA-CUR NPs. (n = 3 for each group). (l) CAT-like activity of HA-CUR NPs (n = 3 for each group)
Radical scavenging and antioxidant capacity of HA-CUR NPs
CUR has strong antioxidant properties, but its low aqueous solubility greatly limits its widespread application. Here, we explored the free radical scavenging ability and antioxidant capacity of HA-CUR NPs. First, the ABTS and DPPH radical assays, which are two radical models widely used for quantifying the antioxidant capacity of biological samples, were adopted to test the radical scavenging ability [18, 19]. The capacity of HA-CUR NPs to scavenge ABTS and DPPH radicals was evaluated by measuring the absorbances at 734 and 517 nm, respectively. As the concentration of HA-CUR NPs increased, the color gradually decreased, and decreased absorbance values were observed (Fig. 1d and e), suggesting the strong ABTS and DPPH radical scavenging abilities of the HA-CUR NPs. Furthermore, the OH radical (·OH) is a physiologically related radical and is involved in various types of cellular and histopathological damage. The ability of HA-CUR NPs to scavenge ·OH was assessed via the methylene blue (MB) assay. Our findings revealed that with the protection of HA-CUR NPs, MB remained blue or faded slightly, indicating that HA-CUR NPs had a strong scavenging ability against ·OH (Fig. 1f). The specific mechanisms of these three assays are schematically presented in Fig. 1g. In addition, 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) were employed to trap •OH and single oxygen, respectively. The authors evaluated the capacity of HA-CUR NPs to scavenge free radicals produced by the Fenton reaction through electron spin resonance (ESR) spectroscopy. A decreased ESR amplitude was observed after treatment with the HA-CUR NPs, suggesting the efficient scavenging ability of the HA-CUR NPs for ·OH and single oxygen (Fig. 1h and i). In summary, these findings indicate that HA-CUR NPs may be effective ROS scavengers because of their strong antioxidant properties.
Mimetic activities of three antioxidant enzymes in the HA-CUR NPs
The effects of HA-CUR NPs on SOD, GSH, and CAT activity were further explored. SOD, an antioxidant enzyme that decomposes superoxide radicals into hydrogen peroxide and oxygen, has been demonstrated to exert remarkable therapeutic effects in uveitis [20]. Furthermore, GSH is a widely present tripeptide thiol and another important intracellular and extracellular antioxidant that plays many important roles in cellular signal transduction processes [21]. H2O2 can be eliminated from the body by decomposition into H2O and O2 by CAT, an iron porphyrin-binding enzyme that plays a vital protective role in organisms. As shown in Fig. 1j, k, and l, the HA-CUR NPs demonstrated SOD, GSH, and CAT mimetic effects in a dose-dependent manner, suggesting that the HA-CUR NPs have excellent antioxidant effects.
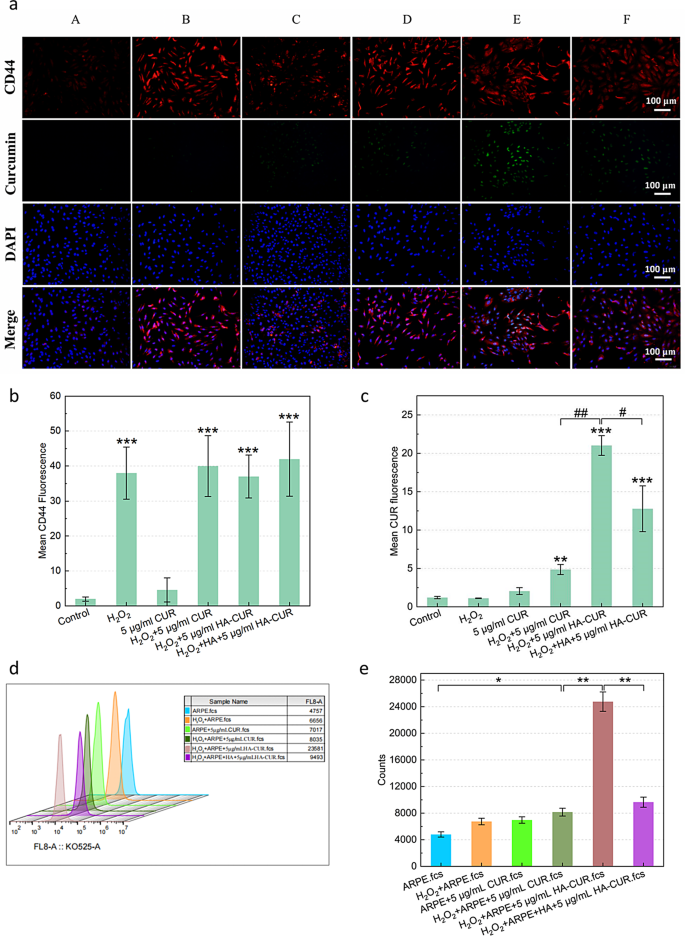
Cellular uptake behavior of HA-CUR NPs and in vitro cytotoxicity of HA-CUR NPs
The pathogenesis and progression of uveitis are closely associated with the impairment of ocular immune privilege, which may be caused by destruction of the integrity of the RPE [22]. Flourescence microscopy was employed to evaluate the ability of HA-CUR NPs to target ARPE-19 cells by identifying their internalization of HA-CUR NPs and free CUR pretreatment with or without H2O2. CUR is represented by the green fluorescence signal, whereas the CD44 receptor is represented by the red signal. ARPE-19 cells that were not treated with H2O2 assimilated minimal amounts of free CUR, as demonstrated in Fig. 2a, and the quantitative analysis results are shown in Fig. 2b and c. The cellular uptake behavior of HA-CUR NPs was more easily observed in ARPE cells pretreated with H2O2 than in those pretreated with free CUR. The following observations were made to ascertain whether the increased cellular absorption of HA-CUR NPs by ARPE-19 cells pretreated with H2O2 was a result of their ability to target the CD44 receptor. Initially, pretreatment of ARPE-19 cells with H2O2 led to greater expression of the CD44 receptor than that in untreated ARPE-19 cells. These findings indicate that the expression of the CD44 receptor may be promoted by severe oxidative stress. ARPE-19 cells were then treated with HA or HA-CUR NPs following H2O2 pretreatment. The absorption of HA-CUR NPs by cells clearly decreased, potentially as a result of the competitive binding of HA to the CD44 receptor, which inhibited NP-CD44 receptor binding. The results were further verified through flow cytometry (Fig. 2d). MTT assays revealed that the activity of ARPE-19 cells was reduced by incubation with higher concentrations of HA-CUR NPs (Figure S3a-b). The relative survival rate of the cells and the proportion of those with a normal morphology decreased (Figure S3c). In summary, these data indicate that the CD44 receptor targeting of HA-CUR NPs may promote the cellular uptake of HA-CUR NPs due to CD44 receptor overexpression in ARPE-19 cells caused by excessive oxidative stress.
(a) Fluorescence images showing CD44 and CUR expression in different groups. A: Control; B: 500 µmol/L H2O2-treated APRE-19 cells for 40 min; C: 5 µg/mL CUR-treated APRE-19 cells for 12 h; D: 500 µmol/L H2O2-treated APRE-19 cells for 40 min + 5 µg/mL CUR-treated APRE-19 cells for 12 h; E: 500 µmol/L H2O2-treated APRE-19 cells for 40 min + 5 µg/mL HA-CUR NP-treated APRE-19 cells for 12 h; F: 500 µmol/L H2O2-treated APRE-19 cells for 40 min + 5 µg/mL HA-treated APRE-19 cells for 4 h + 5 µg/mL HA-CUR NP-treated APRE-19 cells for 12 h. (b) Quantification of the fluorescence intensity of CD44 in different groups. (c) Quantification of the fluorescence intensity of CUR in different groups. (d, e) Flow cytometry analysis of the fluorescence intensity of CUR in different groups. Note: */#, P < 0.05; **/##, P < 0.01; ***/###, P < 0.001
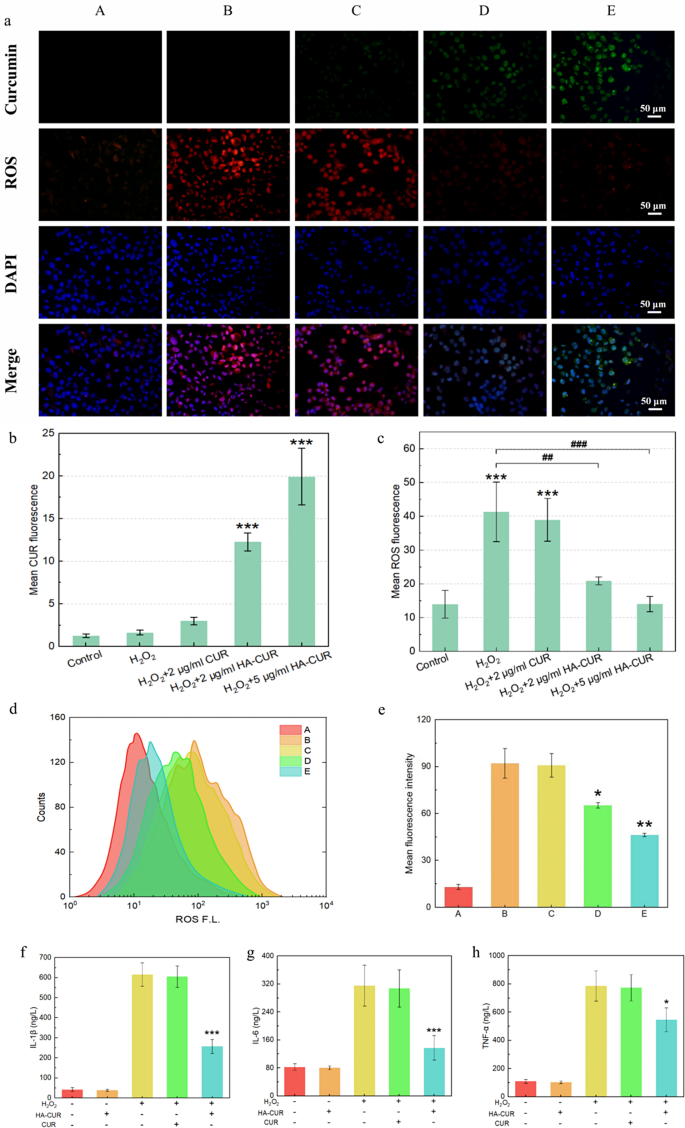
HA-CUR NPs attenuated oxidative stress and inflammatory responses in ARPE-19 cells
Encouraged by their excellent cellular uptake, HA-CUR NPs were evaluated for their ability to safeguard ARPE-19 cells from oxidative stress caused by H2O2. DCFH-DA staining was conducted to visualize intracellular ROS after different interventions. The green fluorescence signal indicates CUR, and the red fluorescence signal represents ROS. Compared with those that were not treated with H2O2, ARPE-19 cells that were pretreated with H2O2 presented a red fluorescence signal, which suggested that the intracellular ROS levels were elevated, as demonstrated by Fig. 3a and the results of the quantitative analysis shown in Fig. 3b and c. When the cells were cocultured with free CUR, significant intracellular ROS and extremely low cellular uptake of CUR were detected. However, the red fluorescence signal decreased significantly, and the green fluorescence signal increased when H2O2-pretreated ARPE-19 cells were cocultured with HA-CUR NPs, suggesting that HA-CUR NPs can cause a dose-dependent reduction in ROS.
(a) Fluorescence images of ARPE-19 cells stained with DFCH-DA and 4′,6-diamidino-2-phenylindole (DAPI) after different treatments. A: Control; B: 500 µmol/L H2O2-treated APRE-19 cells for 40 min; C: 500 µmol/L H2O2-treated APRE-19 cells for 40 min + 2 µg/L CUR-treated APRE-19 cells for 12 h; D: 500 µmol/L H2O2-treated APRE-19 cells for 40 min + 2 µg/mL HA-CUR NP-treated APRE-19 cells for 12 h; E: 500 µmol/L H2O2-treated APRE-19 cells for 40 min + 5 µg/mL HA-CUR NP-treated APRE-19 cells for 12 h. (b) Quantification of the fluorescence intensity of CUR in different groups. (c) Quantification of the fluorescence intensity of ROS in different groups. (d) Flow cytometry quantification of ARPE-19 cells stained with DFCH-DA after different treatments. (e) Mean fluorescence intensity calculated from the flow cytometry data in d; (f) expression level of IL-1β in different groups; (g) expression level of IL-6 in different groups; (h) expression level of TNF-α in different groups. Note: */#, P < 0.05; **/##, P < 0.01; ***/###, P < 0.001
These findings were further verified via flow cytometry (Fig. 3d and e). Furthermore, H2O2-pretreated ARPE-19 cells exhibited markedly increased production of inflammatory cytokines, including IL-1β, IL-6 and TNF-α, which were downregulated by HA-CUR NPs but not by CUR (Fig. 3f, g and h). These results collectively indicate that HA-CUR NPs may mitigate ARPE-19 cell oxidative stress and the inflammatory response.
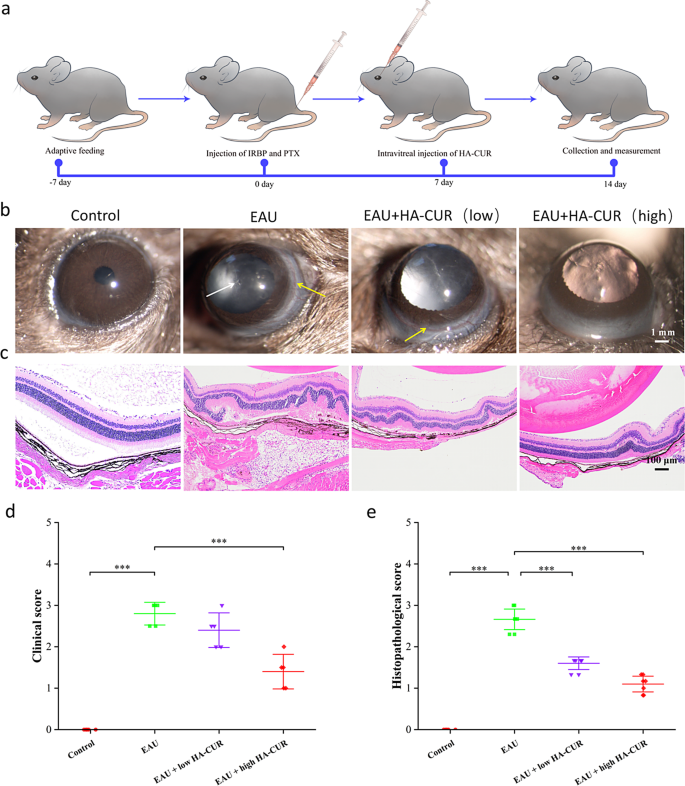
HA-CUR NPs attenuated pathological progression, relieved microvascular damage, and regulated fundus blood flow in vivo
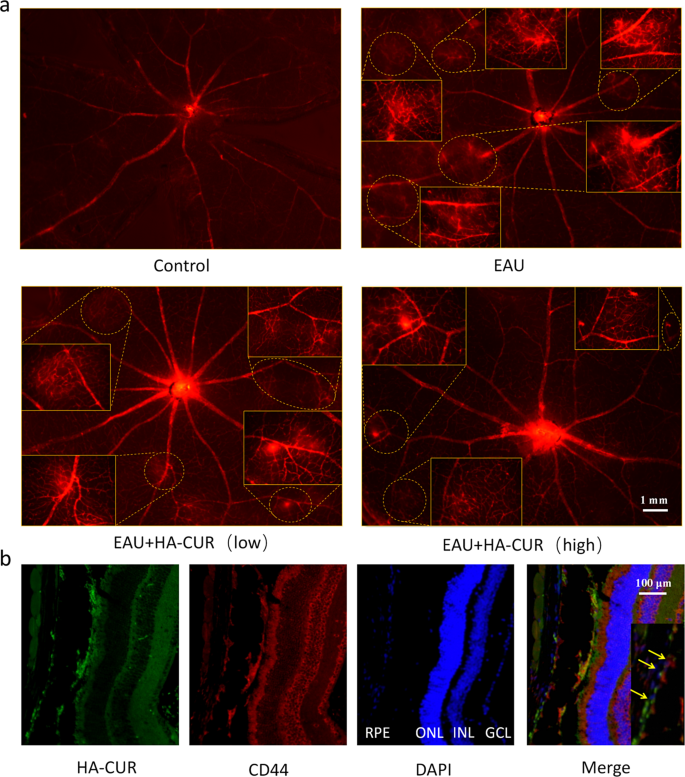
EAU mice’s clinical symptoms and retinal tissue pathological changes were graded by severity according to previous standards [23, 24]. On the basis of the excellent cellular uptake behavior and ability of HA-CUR NPs to protect against oxidative stress damage in vitro, we researched the therapeutic efficacy of HA-CUR NPs in vivo (Fig. 4a). Compared with normal control rats, EAU mice presented significant inflammation in the anterior chamber and severe ciliary and conjunctival hyperemia, whereas EAU mice treated with an intravitreal injection of high HA-CUR NPs presented relieved symptoms (Fig. 4b). H&E staining revealed typical histopathological signs in the EAU model, including significantly increased retinal folds and increased inflammatory cells, which were markedly attenuated in the EAU model mice treated with the intravitreal injection of high concentrations of HA-CUR NPs (Fig. 4c). Clinical and histopathological scores were also assessed (Fig. 4d and e). The effects of HA-CUR NPs on the regulation of retinal vascularity and blood flow in vivo were also thoroughly investigated. Evans blue serves as a diazo dye that quickly and irreversibly binds to plasma ALB and therefore can be used as a highly efficient retinal angiographic agent. As shown in the retinal vascular network images obtained from Evans blue staining in Fig. 5a, massive vascular leakage sites were observed in the retinas of the EAU mice, and this leakage was markedly alleviated in the EAU mice treated with the intravitreal injection of high concentrations of HA-CUR NPs (Figure S4). Conversely, symptoms were not significantly ameliorated in EAU model mice following the intravitreal injection of low concentrations of HA-CUR NPs. Furthermore, immunohistochemistry was implemented to evaluate the targeting of RPE cells by HA-CUR NPs. As shown in Fig. 5b, HA-CUR NPs could be observed in the cytoplasm of RPE cells. The results revealed the colocalization of HA-CUR NPs and CD44-positive RPE cells.
(a) Illustration of the experimental procedure used to establish the experimental autoimmune uveitis (EAU) model. (b) Clinical manifestations of retinas in the different groups were observed via a slit lamp. The white arrows indicate anterior chamber inflammation; the yellow arrows indicate conjunctival and ciliary congestion. (c) HE-stained histopathological images of retinas from the different groups. (d) Clinical score based on the results in (b). (e) Histopathological score based on the results in (C). Note: *, P < 0.05; **, P < 0.01; ***, P < 0.001
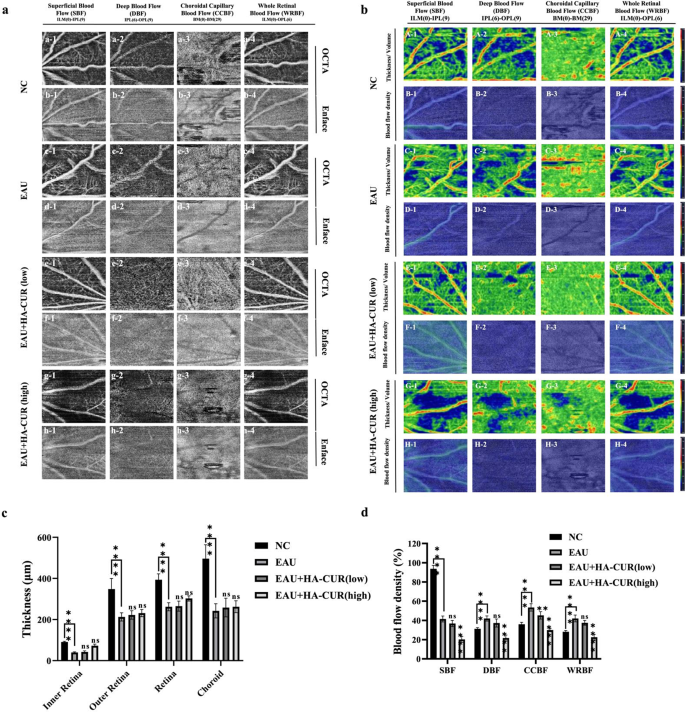
OCTA, a noninvasive fundus vascular imaging technology that is innovative, has been extensively employed to diagnose fundus disorders, including diabetic retinopathy and uveitis. Unlike traditional methods, OCTA does not require the intravenous application of dye, preventing fluorescein leakage [25]. We employed OCTA imaging to quantify the thickness and density of blood flow in each fundus layer in distinct groups of mice. Figure 6a shows the superficial blood flow (SBF), deep blood flow (DBF), choroidal capillary blood flow (CCBF), and whole retinal blood flow (WRBF) of the fundus of the various groups. In contrast to NC mice, EAU mice presented typical retinal vascular morphological anomalies. Conversely, the retinal vascular networks of EAU model mice were normal following intravitreal injections of both low and high concentrations of HA-CUR NPs. Figure 6b shows the OCTA fundus blood flow and thickness/volume maps. Compared with NC mice, EAU mice presented more impaired blood flow signals and uneven vessel morphology across the whole retinal vascular network, indicating significant vascular dysfunction in the fundus of the EAU mice. In contrast, these abnormal symptoms were alleviated in EAU model mice by intravitreal injections of both low and high concentrations of HA-CUR NPs. The quantitative analysis of the SBF, DBF, CCBF, and WRBF layers of the fundus, as illustrated in Fig. 6c and d, revealed a marked decrease in thickness in the EAU group compared with the NC group. Both the EAU mice treated with the intravitreal injection of low- and high-HA-CUR NPs subsequently exhibited remarkable improvements to relatively normal levels. These results indicate that HA-CUR NP treatment markedly attenuated pathological progression, relieved microvascular leakage, and regulated fundus blood flow; therefore, HA-CUR NPs serve as effective agents for retinal protection in EAU.
Optical coherence tomography angiography (OCTA) imaging, blood flow, and thickness of multiple layers of OCTA in the fundus. (a) Superficial blood flow (SBF) (a1-h1), deep blood flow (DBF) (a2-h2), choroidal capillary blood flow (CCBF) (a3-h3), and whole retinal blood flow (WRBF) (a4-h4) of the OCTA panorama in different groups. (b) OCTA fundus thickness/volume (A, C, E, G) and blood flow (B, D, F, H) maps in different groups. (c) Quantification of the thickness of the SBF, DBF, CCBF, and WRBF retinal layers in different groups. (d) Quantification of blood flow in the SBF, DBF, CCBF, and WRBF retinal layers in different groups. Asterisks indicate a significant difference. Note: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001
HA-CUR NPs mitigated oxidative stress and reduced inflammatory responses
HA-CUR NPs have been shown to mitigate oxidative stress in vitro; thus, we investigated whether HA-CUR NPs exert similar effects in vivo. MDA is usually regarded as a marker for lipid peroxidation and oxidative stress [26], whereas SOD is a metalloenzyme that safeguards the organism against oxidative stress [27]. The redox state of uveitis is suggested to be imbalanced, as evidenced by the significantly higher intraocular MDA and reduced intraocular SOD in EAU animals than in normal controls (Fig. 7a-j). Fortunately, the administration of HA-CUR NPs dose-dependently decreased high MDA levels and elevated low SOD levels in EAU mice, whereas treatment with free CUR had a minimal effect on these indicators. Furthermore, given that IL-17, IFN-γ and TNF-α are characteristic inflammatory cytokines that participate in the pathogenesis and development of uveitis [28], we further examined the expression of these three cytokines in the different groups. Compared with NC mice, EAU mice presented markedly greater expression levels of intraocular IL-17, IFN-γ and TNF-α, which were decreased in a dose-dependent manner by treatment with HA-CUR NPs rather than free CUR. Furthermore, we quantified the expression of the CD44 receptor in response to the excessive oxidative stress that was induced in vitro (Fig. 7k, l). The CD44 receptor expression of the EAU mice exceeded that of the normal controls, suggesting a potentially more intense targeted interaction between HA and the receptor. The combination of CUR and HA may increase CUR bioavailability by targeting HA to the CD44 receptor, reducing oxidative stress and inflammatory responses, given that HA-CUR NPs are more efficacious than free CUR is. Considering that HA-CUR NPs are significantly more efficacious than free CUR is, one reasonable conclusion can be drawn that the combination of CUR and HA could markedly increase the bioavailability of CUR, which may be mediated by the specific targeting of HA to the CD44 receptor, thereby efficiently attenuating excessive inflammatory responses and oxidative stress.
Quantification of the serum MDA (a), SOD (b), TNF-α (c), IFN-γ (d), and IL-17 (e) levels and the intraocular MDA (f), SOD (g), TNF-α (h), IFN-γ (i), and IL-17 (j) levels in different groups. (k, l) Expression of the CD44 receptor in control and EAU rats. Note: */#, P < 0.05; **/##, P < 0.01; ***/###, P < 0.001
HA-CUR NPs exerted anti-inflammatory effects by activating the Keap1/Nrf2/HO-1 signaling pathway
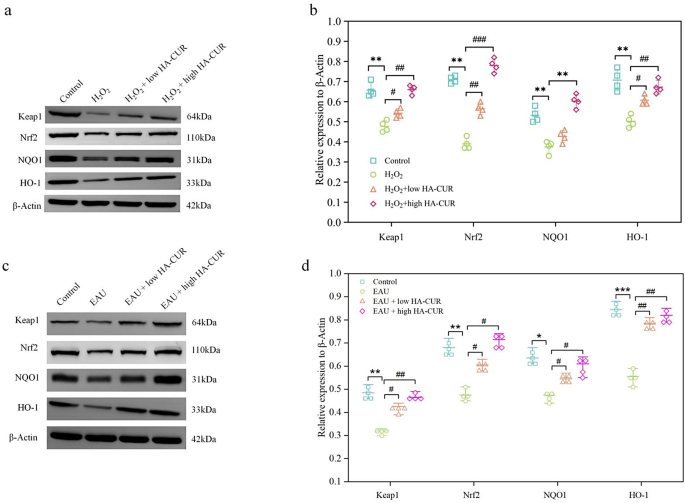
By identifying the potential mechanism behind the prominent oxidative stress protection of HA-CUR NPs, we may be able to better understand the therapeutic benefits of HA-CUR NPs and optimize their clinical application. To further explore the molecular mechanism of HA-CUR NPs, we conducted transcriptome sequencing analysis on ARPE-19 cells treated with different interventions. The Molecular Signatures Database C2 database was used to perform GSEA on the DEGs, and the top 20 results are illustrated in Fig. 8a. The majority of genes whose expression was significantly upregulated by HA-CUR NPs were associated with inflammation, immunology, and oxidative stress, including REACTOME_KEAP1_NFE2L2_PATHWAY, a classic pathway that is involved in the antioxidative stress response and safeguards cells from oxidative damage. Additional enrichment analysis confirmed the activation of the Keap1/Nrf2 signaling pathway (Fig. 8b and c). A gene expression heatmap of the Keap1/Nrf2 signaling pathway revealed that several genes in the Keap1/Nrf2 signaling pathway, including GSTM5, GSTO2, MGST1, KEAP1, HMOX1, and NFE2L2, were upregulated by the HA-CUR NPs (Fig. 8d and e), which promoted the expression of anti-inflammatory and antioxidant-related proteins to resist oxidative stress damage. In addition, the top 5 potential signaling pathways and the top 6 DEGs revealed by transcriptome sequencing analysis are shown in Fig. 8f. As one of the most vital antioxidant axes in physiological and pathological processes, the activation of Nrf2 signaling could contribute to a series of antioxidant responses, thereby protecting cells against oxidative stress damage [29, 30]. Specifically, Nrf2 is a transcription element that promotes the transcription and expression of various major antioxidative enzymes, including heme oxygenase-1 (OH-1) and NAD(P)H quinone oxidoreductase 1 (NQO1), by binding to antioxidation stress reaction sites [30, 31]. In numerous cell lines, including RPE cells, natural products have been shown to alter the Keap1/Nrf2/HO-1 signaling pathway [32]. Notably, Nrf2 was found to be closely related to the development of uveitis, serving as a potential intervention target in the modulation of redox hemostasis [33]. Western blotting was used to determine whether HA-induced CUR NPs can safeguard ARPE-19 cells and EAU model mice from oxidative stress-induced damage by activating the Keap1/Nrf2/HO-1 signaling pathway. The protein expression levels of Keap1, Nrf2, HO-1, and NQO1 in ARPE-19 cells pretreated with hydrogen peroxide were substantially lower than those in the control group. However, the H2O2-induced effects on the Keap1/Nrf2/HO-1 signaling pathway were reversed by HA-CUR NPs in a dose-dependent manner, as illustrated in Fig. 9a and b. Furthermore, in contrast to those in the control group, the protein expression levels of Keap1, Nrf2, HO-1, and NQO1 in the EAU group were substantially lower. On the other hand, HACUR NPs were still capable of reversing the changes in protein expression levels in a dose-dependent manner (Fig. 9c and d). These findings indicate that Keap1/Nrf2/HO-1 induces the protective influence of the HA CUR NPs. These results suggest that the Keap1/Nrf2/HO-1 signaling pathway contributes significantly to the anti-inflammatory effect of the HA CUR NPs. Curcumin is a natural activator of Nrf2 [34]. Mechanistic studies have shown that curcumin induces conformational changes in Keap1 and the ubiquitination of Nrf2 by binding to Keap1Cys151 [35]. However, the effects of curcumin on the protein expression of Nrf2 in cells subjected to oxidative stress are not entirely consistent. Liu et al. confirmed that pretreatment with curcumin did not increase the expression of total Nrf2 in cells subjected to oxidative stress stimulation [36]. These findings are inconsistent with our findings. However, some studies have shown that curcumin can increase the protein expression of Nrf2 in cells subjected to various oxidative stresses [35, 37]. The concentration of curcumin used and the duration of its action may be key factors influencing the level of Nrf2 expression.
(a) The top 20 results of GSEA enrichment analysis of the DEGs via the Molecular Signatures Database C2 database. (b, c) Running enrichment score of the Keap1/Nrf2 signaling pathway. (d, e) Gene expression heatmap of the Keap1/Nrf2 signaling pathway regulated by HA-CUR NPs. (f) Protein levels of Keap1, Nrf2, HO-1, and NQO1 in ARPE-19 cells after different treatments. h, i) Protein levels of Keap1, Nrf2, HO‐1, and NQO1 in rats in different groups
Assessment of biocompatibility in vivo
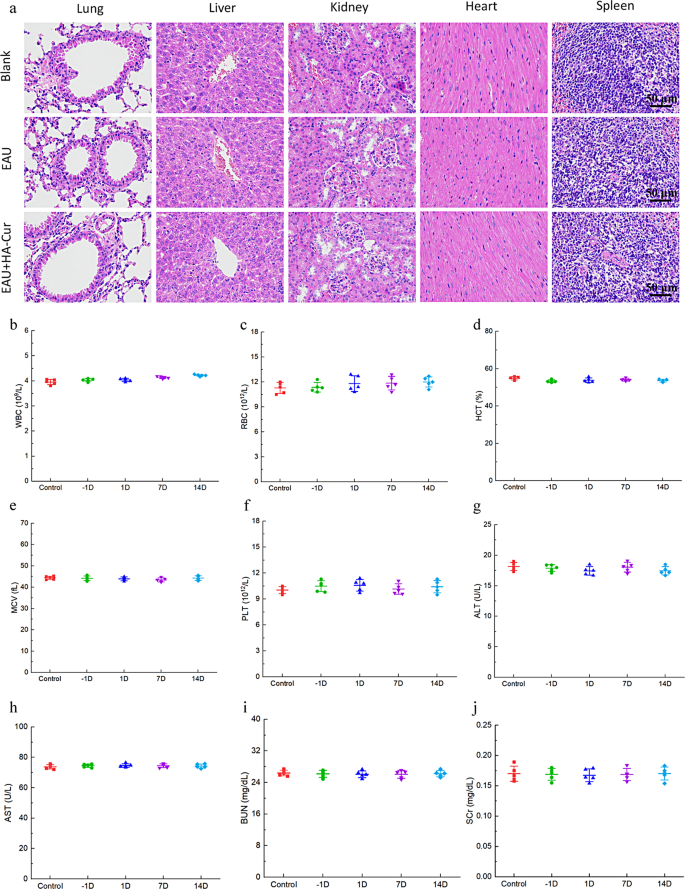
The biocompatibility of HA-CUR NPs in vivo was assessed through H&E staining of important organs, including the heart, liver, spleen, lung, and kidney. As shown in Fig. 10a-j, no remarkable lesions, hemorrhage, or necrosis were detected in these major organs. No substantial changes in liver or renal function were observed in any of the mice in the present study, as determined by ALT, AST, BUN, and SCR in the serum. The WBC, RBC, PLT, MCV, and HCT were also measured and demonstrated no significant changes. No obvious lesions were found in the retinal tissue after the HA-CUR NPs were injected into the eye. (Figure S5a). We further examined three inflammatory cytokines to evaluate the toxicity of HA-CUR NPs to intraocular tissues. As shown in Figure S5b, the expression levels of IL-17, IFN-γ and TNF-α in the eyes of the mice injected with HA-CUR NPs did not significantly differ from those in the control group. Therefore, the HA-CUR NPs synthesized in the present study have the potential to serve as safe and efficacious agents for preventing the progression of EAU.
In vivo biocompatibility of HA-CUR NPs. (a) Images of H&E-stained heart, liver, spleen, lung, and kidney tissues from the different groups. (b–f) Quantification of white blood cells (WBCs), red blood cells (RBCs), platelets (PLTs), mean corpuscular volume (MCV), and hematocrit (HCT) in the different groups. (g–j) Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and serum creatinine (SCR) levels in the different groups